Put the following in order of increasing standard molar entropy: a solid, a liquid, and a gas. Standard molar entropy is a measure of the randomness or disorder of a system, and it is typically expressed in units of J/(mol·K).
The higher the standard molar entropy, the more disordered the system.
The standard molar entropy of a substance depends on a number of factors, including its molecular structure, its physical state, and its temperature and pressure. In general, gases have higher standard molar entropy values than liquids, and liquids have higher standard molar entropy values than solids.
This is because gases are more disordered than liquids, and liquids are more disordered than solids.
Standard Molar Entropy
Standard molar entropy ( S°) is a measure of the randomness or disorder of a system at standard conditions (298 K and 1 atm). It is expressed in units of joules per mole per Kelvin (J/mol·K). The higher the standard molar entropy, the more disordered the system.
Standard molar entropy is related to the number of possible microstates of a system. A microstate is a specific arrangement of the particles in a system. The more microstates a system has, the more disordered it is. This is because a system with a large number of microstates has a greater chance of being in a high-energy state, which is a more disordered state.
Factors Affecting Standard Molar Entropy
Several factors influence the standard molar entropy of a substance, including:
- Molecular structure and complexity:Molecules with more complex structures and more degrees of freedom have higher standard molar entropies.
- Physical state:Gases have higher standard molar entropies than liquids, which in turn have higher standard molar entropies than solids.
- Temperature and pressure:Standard molar entropy increases with increasing temperature and decreases with increasing pressure.
Ordering Substances by Standard Molar Entropy
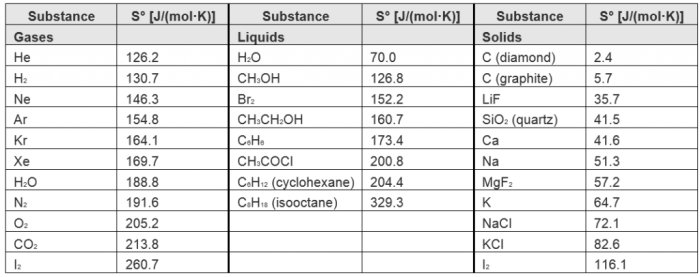
Substances can be ordered based on their increasing standard molar entropy. The following table shows the standard molar entropy values for some common substances:
| Substance | Standard Molar Entropy (J/mol·K) |
|---|---|
| Hydrogen gas (H2) | 130.6 |
| Water (liquid) | 69.9 |
| Carbon dioxide (gas) | 213.6 |
| Sodium chloride (solid) | 72.1 |
Applications of Standard Molar Entropy
Standard molar entropy has several practical applications, including:
- Predicting the spontaneity of reactions:The change in standard molar entropy ( ΔS°) for a reaction can be used to predict whether the reaction is spontaneous or not.
- Determining the feasibility of processes:Standard molar entropy can be used to determine the feasibility of processes, such as the separation of a mixture or the production of a new material.
- Designing efficient energy systems:Standard molar entropy can be used to design efficient energy systems, such as heat engines and refrigerators.
Limitations and Considerations, Put the following in order of increasing standard molar entropy
There are some limitations and considerations associated with using standard molar entropy as a measure of disorder. For example, standard molar entropy does not take into account the interactions between particles. In some cases, these interactions can lead to a decrease in disorder, even if the standard molar entropy increases.
FAQ Explained: Put The Following In Order Of Increasing Standard Molar Entropy
What is standard molar entropy?
Standard molar entropy is a measure of the randomness or disorder of a system, and it is typically expressed in units of J/(mol·K).
What factors affect standard molar entropy?
The standard molar entropy of a substance depends on a number of factors, including its molecular structure, its physical state, and its temperature and pressure.
How can standard molar entropy be used?
The standard molar entropy of a substance can be used to predict the spontaneity of reactions and to determine the feasibility of processes. It can also be used to design efficient energy systems.