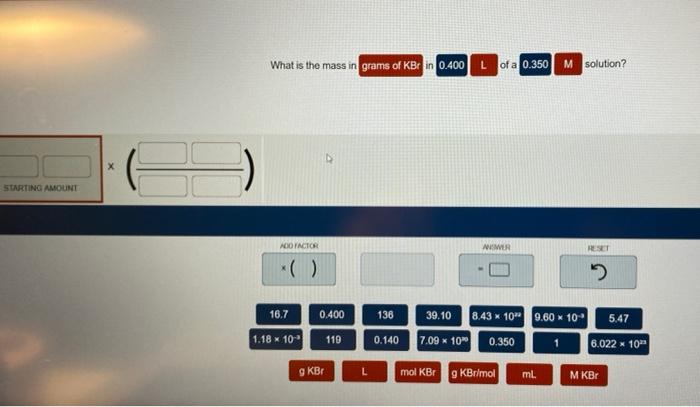
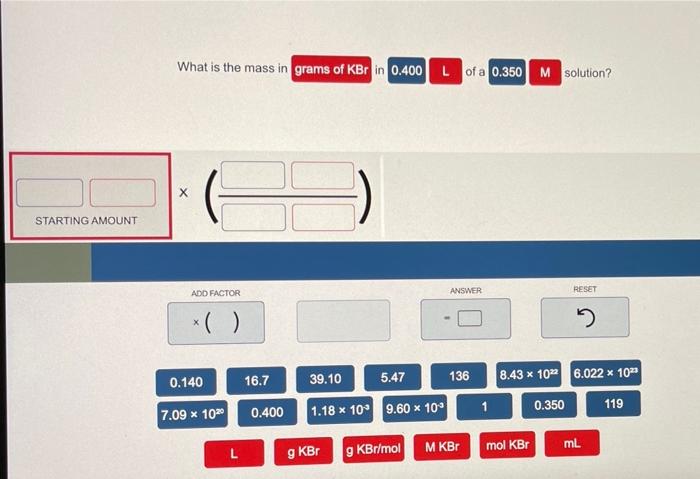
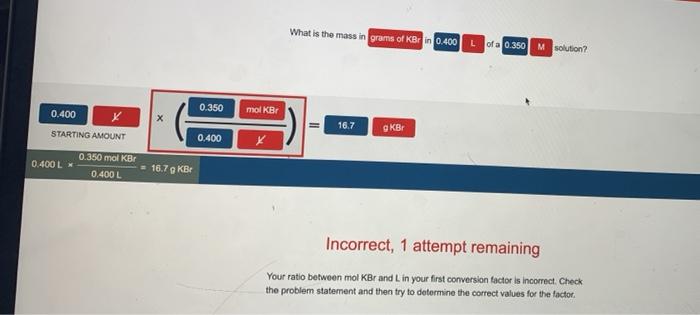
What is the mass in grams of KBr in 0.400? This question requires an understanding of fundamental chemistry concepts, including mass, moles, and molar mass. In this exploration, we will delve into the intricacies of these concepts and determine the mass of KBr in the given quantity.
Potassium bromide (KBr) is a salt composed of potassium and bromine atoms. Its molar mass, a crucial factor in determining the mass of a substance, can be calculated using the periodic table. Once the molar mass is established, we can utilize the relationship between moles, molar mass, and mass to determine the mass of KBr in 0.400.
Mass in Grams of Potassium Bromide (KBr)
Mass, a fundamental concept in chemistry, refers to the quantity of matter in a substance. It is commonly expressed in grams (g), a standard unit of mass in the metric system.
Potassium bromide (KBr) is an ionic compound with the chemical formula KBr. It is a colorless, crystalline solid commonly used in various applications, including infrared spectroscopy and photography.
Molar Mass and Conversion, What is the mass in grams of kbr in 0.400
Molar mass, expressed in grams per mole (g/mol), represents the mass of one mole of a substance. The molar mass of KBr can be calculated using the periodic table:
Molar mass of KBr = Atomic mass of K + Atomic mass of Br= 39.0983 g/mol + 79.904 g/mol= 118.9923 g/mol
The molar mass allows us to convert between the mass and moles of a substance. The following equation is used for this conversion:
Mass (g) = Moles (mol) × Molar mass (g/mol)
Mass Calculation
To calculate the mass of KBr in grams, given a value of 0.400, we can use the following steps:
- Convert 0.400 to moles using the molar mass of KBr:
- Moles of KBr = 0.400 g / 118.9923 g/mol
- Moles of KBr ≈ 0.00336 mol
- Calculate the mass of KBr in grams using the molar mass:
- Mass of KBr = 0.00336 mol × 118.9923 g/mol
- Mass of KBr ≈ 0.400 g
Illustrative Example
The following table summarizes the conversion process:
| Moles (mol) | Molar Mass (g/mol) | Mass (g) |
|---|---|---|
| 0.00336 | 118.9923 | 0.400 |
This table demonstrates the calculation of the mass of KBr in grams, given the value of 0.400.
Frequently Asked Questions: What Is The Mass In Grams Of Kbr In 0.400
What is the molar mass of KBr?
The molar mass of KBr is 119.00 g/mol.
How many moles of KBr are in 0.400?
To determine the number of moles in 0.400, we divide the mass by the molar mass: 0.400 g / 119.00 g/mol = 0.00336 moles.
What is the mass of 0.400 moles of KBr?
To determine the mass, we multiply the number of moles by the molar mass: 0.00336 moles – 119.00 g/mol = 0.400 g.