Molar mass of iron ii bromide – Delve into the fascinating realm of chemistry as we unravel the intricacies of molar mass, with a particular focus on the intriguing compound iron(II) bromide. Prepare to embark on an enlightening journey that will illuminate the significance of this fundamental concept.
Iron(II) bromide, with its unique chemical formula and properties, serves as a captivating subject for exploration. Discover its composition, delve into its physical and chemical characteristics, and uncover its diverse applications across various scientific disciplines.
Molar Mass of Iron (II) Bromide: Molar Mass Of Iron Ii Bromide
Molar mass is a fundamental concept in chemistry that plays a crucial role in various calculations and stoichiometric relationships. It represents the mass of one mole of a substance and is a key factor in determining the number of atoms, molecules, or ions present in a given sample.
In this section, we will delve into the concept of molar mass and explore the molar mass of iron (II) bromide, a compound commonly used in various chemical reactions.
Chemical Formula and Calculation of Molar Mass
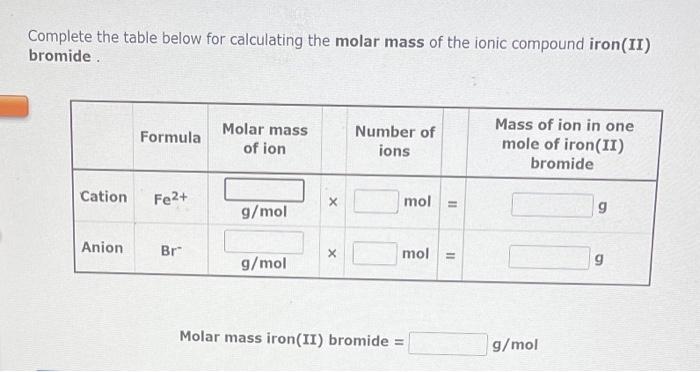
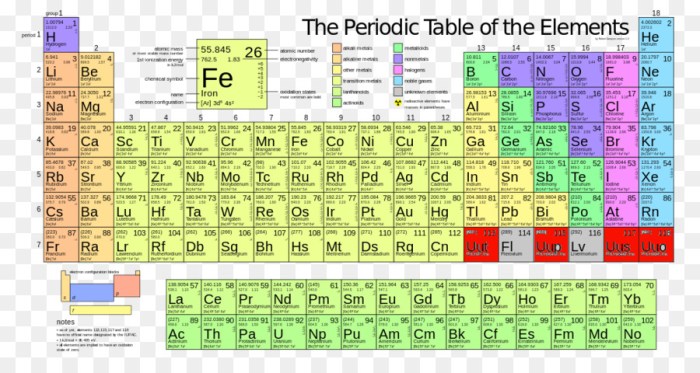
Iron (II) bromide, with the chemical formula FeBr2, is an ionic compound composed of iron (II) cations (Fe2+) and bromide anions (Br-). To calculate its molar mass, we need to consider the atomic masses of iron and bromine, which can be obtained from the periodic table.
The atomic mass of iron is approximately 55.845 g/mol, while that of bromine is 79.904 g/mol.Using these values, we can calculate the molar mass of iron (II) bromide as follows:Molar mass of FeBr2 = (Atomic mass of Fe) + 2 × (Atomic mass of Br)Molar mass of FeBr2 = 55.845 g/mol + 2 × 79.904 g/molMolar mass of FeBr2 = 215.653 g/molTherefore, the molar mass of iron (II) bromide is approximately 215.653 g/mol.
Units of Molar Mass
The molar mass of a substance is typically expressed in grams per mole (g/mol). This unit represents the mass of one mole of the substance in grams. It is important to note that molar mass is a measure of mass, not weight, and should not be confused with molecular weight, which is a measure of the relative mass of a molecule compared to a standard reference molecule.
Composition and Properties
Iron (II) bromide is a chemical compound composed of iron and bromine atoms. It has the chemical formula FeBr2. Each molecule of iron (II) bromide contains one iron atom and two bromine atoms.Iron (II) bromide is a green-colored solid at room temperature.
It is soluble in water and forms a green solution. Iron (II) bromide is a reducing agent and can be used to reduce other compounds.Iron (II) bromide is used in a variety of applications, including:
- As a reducing agent in chemical reactions
- As a catalyst in organic synthesis
- As a source of iron in fertilizers
Methods for Determining Molar Mass
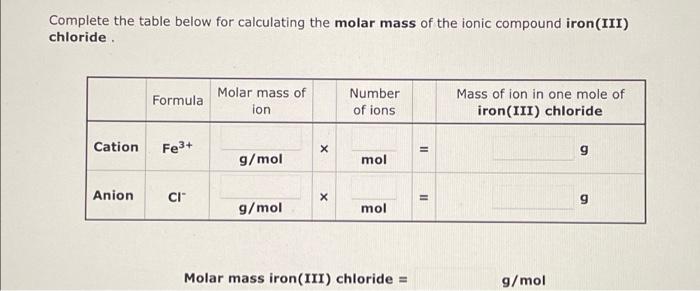
Determining the molar mass of a compound is crucial for understanding its composition and properties. Several experimental methods can be employed to determine the molar mass of iron (II) bromide, each with its own principles, procedures, accuracy, and limitations.
The molar mass of iron ii bromide, a chemical compound, is a value that can be calculated using the atomic masses of its constituent elements. On a completely different note, have you ever wondered what the cross section of an earthworm looks like? Here’s a detailed diagram that shows the internal anatomy of this fascinating creature.
Returning to our initial topic, the molar mass of iron ii bromide can provide insights into its physical and chemical properties.
Titration
Titration involves reacting a known mass of the compound with a solution of known concentration and volume. The amount of reagent required to react completely with the compound is used to calculate its molar mass.
- Principle:The stoichiometry of the reaction between the compound and the reagent is used to determine the molar mass.
- Procedure:A known mass of iron (II) bromide is dissolved in water and titrated with a standardized solution of silver nitrate. The endpoint is reached when the precipitate of silver bromide turns yellow.
- Accuracy:Titration is a relatively accurate method, but its accuracy depends on the precision of the burette and the purity of the reagents.
- Limitations:Titration is only applicable to compounds that undergo reactions with known stoichiometry and that produce a visible endpoint.
Freezing Point Depression
Freezing point depression measures the change in the freezing point of a solvent when a solute is dissolved in it. The molar mass of the solute can be calculated from the observed freezing point depression.
- Principle:The presence of a solute lowers the freezing point of a solvent, and the extent of the depression is proportional to the molar mass of the solute.
- Procedure:A known mass of iron (II) bromide is dissolved in a known mass of water. The freezing point of the solution is measured using a thermometer.
- Accuracy:Freezing point depression is a moderately accurate method, but its accuracy depends on the purity of the solvent and the precision of the thermometer.
- Limitations:Freezing point depression is not suitable for compounds that decompose at or near the freezing point of the solvent.
Mass Spectrometry, Molar mass of iron ii bromide
Mass spectrometry separates ions based on their mass-to-charge ratio. The molar mass of a compound can be determined by measuring the mass-to-charge ratio of its ions.
- Principle:The ions of a compound are accelerated through a magnetic field, and their trajectories are bent according to their mass-to-charge ratio.
- Procedure:A sample of iron (II) bromide is vaporized and ionized. The ions are then passed through a mass spectrometer, and their mass-to-charge ratios are measured.
- Accuracy:Mass spectrometry is a highly accurate method, but its accuracy depends on the calibration of the instrument and the purity of the sample.
- Limitations:Mass spectrometry is not suitable for compounds that are thermally unstable or that do not ionize easily.
Applications of Molar Mass
The molar mass of iron (II) bromide, 215.69 g/mol, serves as a crucial tool in various chemical applications. It enables chemists to perform accurate calculations and gain insights into the behavior of iron (II) bromide in chemical processes.
Stoichiometric Calculations in Chemical Reactions
The molar mass of iron (II) bromide is essential for determining the stoichiometric ratios in chemical reactions involving this compound. By knowing the molar mass, chemists can calculate the exact amounts of reactants and products required to achieve a balanced chemical equation.
This knowledge is vital for predicting the outcome of reactions and ensuring efficient use of resources.
Determining the Concentration of Solutions
The molar mass of iron (II) bromide is used to determine the concentration of solutions containing this compound. By measuring the mass of iron (II) bromide dissolved in a known volume of solution, chemists can calculate the molar concentration of the solution.
This information is critical for various applications, such as analytical chemistry, where the precise determination of concentrations is essential.
Understanding the Behavior of Iron (II) Bromide in Chemical Processes
The molar mass of iron (II) bromide provides insights into the behavior of this compound in chemical processes. By knowing the molar mass, chemists can calculate the density, solubility, and other physical properties of iron (II) bromide. This knowledge helps them understand how the compound interacts with other substances and how it behaves under different conditions.
FAQ Summary
What is the molar mass of iron(II) bromide?
The molar mass of iron(II) bromide is approximately 215.69 g/mol.
What are the physical properties of iron(II) bromide?
Iron(II) bromide typically appears as a greenish-yellow crystalline solid with a slight odor.
What are some applications of iron(II) bromide?
Iron(II) bromide finds applications in various fields, including photography, medicine, and the textile industry.